REPORT Results of the season December 2017 – May 2018 agreement «Global influenza hospital-based surveillance network” - Branch: MOSCOW, Russian Federation
Ministry of HEALTH OF Russian Federation
D.I. Ivanovsky Institute of Virology FSBI “N.F. Gamaleya NRCEM”, Moscow, Russian Federation
REPORT
Results of the season December 2017 – May 2018
agreement
«Global influenza hospital-based surveillance network” - Branch: MOSCOW, Russian Federation
Address: Gamaleya street 18, 123098, Moscow, Russia
Tel.: +7 (499) 190-3046
Fax: +7 (499) 190-2867
e-mail: elena-burtseva@yandex.ru; elena_burceva@mail.ru
Director
FSBI “N.F. Gamaleya NRCEM” MH RF,
academician RAS Gintsburg A.L.
Responsible person
Chief of influenza etiology and
epidemiology laboratory Burtseva E.I.
FSBI “D.I. Ivanovsky Institute of Virology” Ministry of Health of Russian Federation is a scientific research organization in the field of virology and epidemiology of influenza, National Influenza center (NIC) collaborating with WHO. In October 15, 2014 it has been joined to FSBI “Institute of Microbiology and Epidemiology named after N.F. Gamaleya” Ministry of Health of Russian Federation and was included in the structure of FSBI “National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya” (“N.F. Gamaleya NRCEM”). N.F. Gamaleya NRCEM annually confirms its status by taking part in the International External Quality Assessment Program (PCR part) for monitoring and strengthening the quality of diagnostic capacity of the Global Influenza Surveillance and Response System (GISRS) and Panel testing in a Quality Control System (isolation and typing/subtyping of viruses). Twice a year N.F. Gamaleya NRCEM presents the samples of epidemic strains to WHO to make solution of influenza vaccines composition by experts of vaccine Composition Meeting (VCM). Since 2011, N.F. Gamaleya NRCEM joined to the GIHSN.
Study setting and population. The study took place in one from four hospitals for infectious diseases in Moscow, FBIH “Clinical Hospital for Infectious Diseases №1” of Health Department of Moscow (CHID), which collaborates with IIV “N.F. Gamaleya NRCEM” in studies of influenza evolution, biological and genetic properties to make recommendation for vaccines and antivirals efficacy. It covers about 15 million citizens and guests of Moscow. CHID specializes on virus infectious diseases mainly, which are caused by influenza and non-influenza respiratory viruses, enteroviruses, hepatitis B and C, rotavirus, AIDS and etc. CHID presents its service (diagnostic, treatment) to 27-30 thousands of patients a year. It has 706 beds, 38% from all beds for patients with infectious diseases in Moscow. The wards for pregnants (69 beds) with virus infections and 12 beds in intensive care unit (ICU) are the most important advantages of this hospital. It is located not far from “N.F. Gamaleya NRCEM”, that allowed to transport all specimens daily, in proper conditions and in time.
6 wards for respiratory diseases (316 beds) participated in the GIHSN. Patients hospitalized in these wards were monitored three days a week – Tuesday, Wednesday and Thursday. There were: 120 from 485 beds for adults, 113 from 231 beds for children (53 – for children of 0-3 y.o. and 60 – for children of 3-14 y.o.), all 69 beds for pregnant women and all 12 beds for patients in intensive care wards. Age of hospitalized patients ranged from 0 to 90 y.o. Patients were interviewed face to face according to the Questionnaire of GIHSN.
Sample management and laboratory procedures. Nasopharyngeal and pharyngeal swabs (for subjects 14 years old or older) or nasopharyngeal and nasal swabs (for subjects less than 14 years of age) have been obtaining from each included patient and have been sending immediately to IIV “N.F. Gamaleya NRCEM”, where they have been testing during 24-48 hours or frizzing at -20ºC (for probes which were taken during weekend).
Commercially available RT-PCR assays (Russia) were used to detect influenza A (subtypes H3 and H1pdm09) and influenza B (Yamagata and Victoria lineages) viruses in swabs: AmpliSens Influenza virus A/B-FL, AmpliSens Influenza virus A-type-FL (H1N1 and H3N2), AmpliSens Influenza virus А/H1-swine-FL (H1N1pdm09). Part sequencing of hemagglutinin (HA) was made for some influenza A(H3N2) positive samples. Also CDC primers and probes were used for testing some samples.
The isolation of epidemic strains was made from influenza-positive swabs using MDCK (influenza B virus) and MDCK-SIAT1 (influenza A(H3N2) virus). To study of antigenic properties HI test with reference sera to recommended vaccine strains of 2017-2018 epidemic season compositions was used. For some of them the sequencing of hemagglutinin (HA) was made to detect their genetic relationship to reference virus.
Limit number (from vaccinated and unvaccinated patients) of sera were studied by HI test to detect the level of specific antibodies to both of vaccine and epidemic strains during the first days of hospitalization.
Study process and results.
The season 2017-2018 was unique and different from the previous 2016-2017 season and characterized rather late start. The first influenza cases were detected on 3rd week of 2018. That time the fieldwork has been opened and screening of patient has been started. The fieldwork lasted 18 weeks; it is much different compared with the previous season, which lasted 23 weeks. Therefore, the study 2017-2018 season lasted from week 3, 2018 (16 January, 2018) till week 20, 2018 (18 May, 2018). Totally, 1733 hospitalized patients were screened for eligibility to participate in the study. From them, 1433 (83%) patients were eligible and 344 (17%) were excluded. Weekly admission by screening of the patients is presented on the Figure 1.
The main reasons for excluding patients were: the admission to the hospital after 7 days of the illness onset (34%), unable to communicate (31%), the discharge from the hospital staying less than 48 hours (28%), patients hospitalized in the last 30 days (7%), not having ILI case (6%), not given consent (4%).
Among the patients who were included to the study totally 552 (39%) had positive results on influenza viruses (Figure 2), among them: 201 (36.4%) were caused by influenza A(H3N2); 163 (29.5%) - A(H1N1)pdm09; 130 (23.5%) – B/Yamagata; 4 – B/Victoria. Also, there were 11 cases of influenza A no-subtyped and 43 cases of influenza B no-subtyped as well.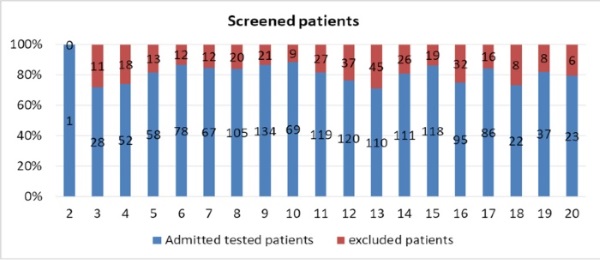
Figure 1. Weekly admission of hospitalized patients during 2017-2018, Moscow
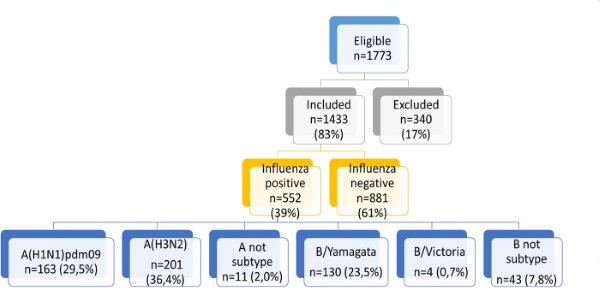
Figure 2. Influenza virus detections among patients in season 2017-2018, Moscow site.
The epidemic 2017-2018 has started due to revealing strains of influenza B/Yamagata-like virus in January 2018. It is definitely untypical beginning of influenza season as commonly influenza B virus has been active in springtime after the first peak of epidemic. Almost all cases of influenza B virus were belonged to influenza B/Yamagata-like lineage. Only four cases of influenza B/Victoria-like was detected through whole season 2017-2018. Influenza A(H1N1)pdm09 and A(H3N2) appeared on the week 6, 2018 and circulated till the end of the season. The highest epidemic activity was during 9th - 15th weeks 2018 according to the highest hospitalizations at that time (end of February-beginning of April). The sharp decrease of admissions was associated with public holiday (Figure 3).
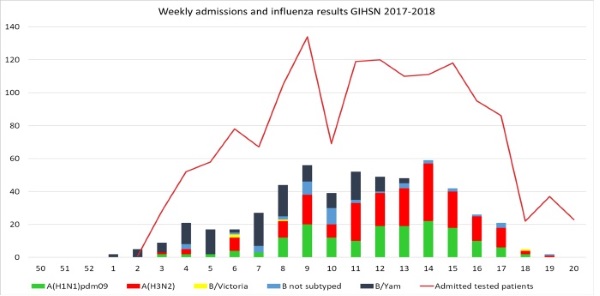
Figure 3. The dynamics of hospitalization and the number of influenza virus detections among patients during 2017-2018, Moscow site
The different aged groups were included in the study, among them: children under 4 y.o. – 569, children 5-14 y.o. – 157, adults 15-64 y.o – 317, pregnants – 318 and elderly (65 y.o. and older) – 73 (Table 1). Meantime, there were 63% (902) females and 37% (531) males among admitted patients.
Influenza viruses were detected among all age groups. However, pregnants, elderly and school children 5-14 y.o. are an especially groups suffered by influenza infection (Figure 4). Totally, influenza A(H3N2) virus was the most common in risk groups, such as elderly (27.4%) and pregnant women (24.5%). The highest percent of influenza A(H1N1)pdm09 was detected in children less than 5 years old (13.2%). Meantime influenza B virus was detected more frequently in age group of children 5-14 y.o. (20.4%).
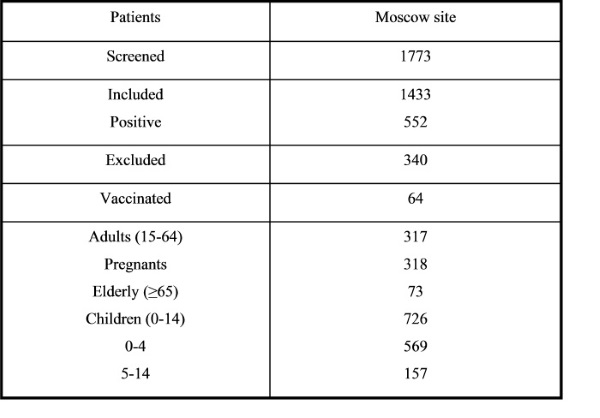
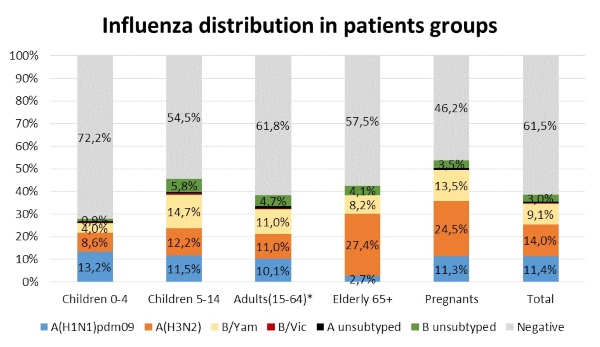
Figure 4. Influenza virus type/subtype distribution in hospitalized patients of different aged groups during 2017-2018
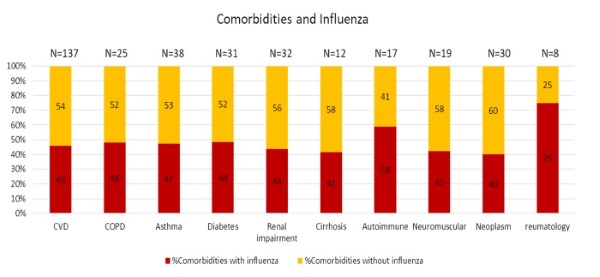
Figure 5. Influenza virus detection in patients with comorbidities during 2017-2018
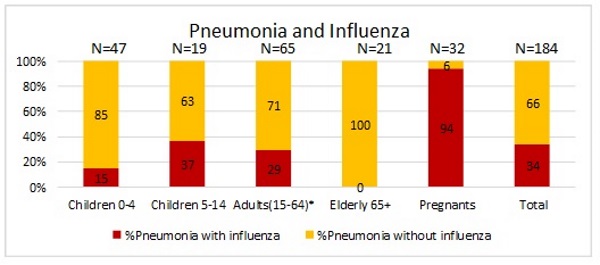
Figure 6. Frequency of pneumonia as severe complication of influenza in different groups of hospitalized patients during 2017-2018.
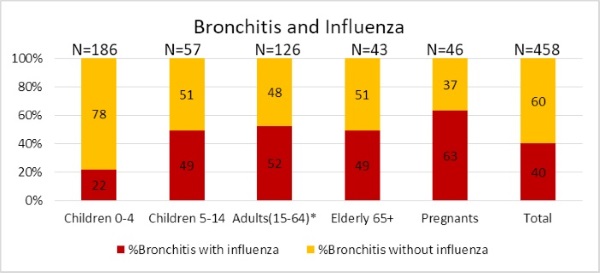
Figure 7. Frequency of bronchitis as complication of influenza and other ARVI in different groups of hospitalized patients during 2016-2017
All clinical symptoms were found among patients with confirmed influenza (fever – 70%, headache – 43%, malaise – 65%, myalgia – 24%, cough – 66%, sore throat – 33% and breath – 10%). Interestingly, hospitalized patients complained the most on fever 38-39 degrees (51%). Fever 39-40 was registered in 46% patients and 37-38 – in 35%.
In the Table 2 the results for influenza virus detection in admitted patients in regards to their smoking habits are presented. In groups of smokers (20% from all) the positive cases of influenza virus infection were detected less in comparing with never smokers (15% vs 23% and 74% vs 71%, accordingly), but there was more influenza cases among, who never smoked or gave up to smoke.
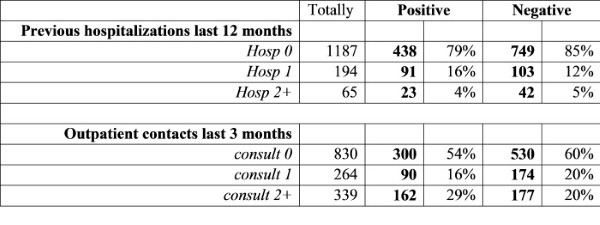
In Table 3 the results for influenza virus detection in patients in regards to numbers of General practitioner consultations (GP) in last 3 months and Hospitalizations last 12 months are presented. Patients with one time of previous hospitalizations (16%) had a little bit more often influenza than other patients. Meantime, influenza was found more frequently in patients who had more than 2 cases of GP consultations in last 3 months before current admission to the hospital. This was observed basically with pregnants.
Table 2
Results for influenza in patients in regards to smoking habits during 2017-2018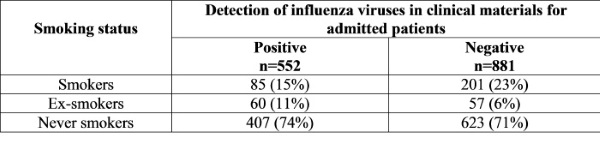
Table 3
Results for influenza in patients in regards to hospitalizations and GP consultations in 2017-2018
The results of influenza virus detection in aged groups of hospitalized patients who were vaccinated are presented on Figure 8. There were 63 (4,4% from all included) vaccinated patients and influenza cases were revealed in 26 (41% from vaccinated and 4,7% from all positive on influenza), among them: A(H1N1)pdm09 – 5, A(H3N2) – 12, A no-subtype - 1 and B - 8 patients. The most cases of influenza were found in vaccinated pregnants (60%) and schoolchildren 5-14 (53%).
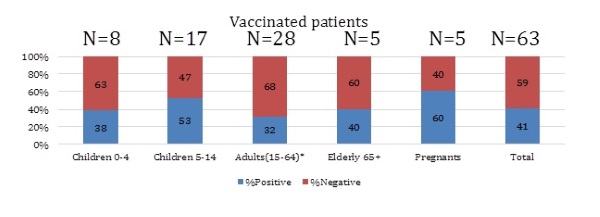
Figure 8. The frequency of influenza virus detection in different aged groups of vaccinated patients
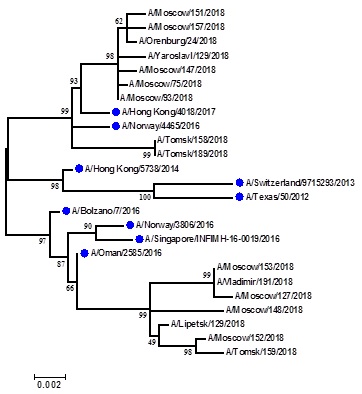
Picture 9. Phylogenetic comparison of HA1 genes of epidemic strains of influenza A(H3N2) virus, isolated from hospitalized patients during season 2017-2018
Key aspects from the season:
Conclusion: the season 2017-2018 was unique and characterized by co-circulating of all types of influenza viruses population of that were presented as strains like vaccine viruses as the new genetic group (A(H3N2)) and another evolutionary line (B/Yamagata-like). The start of epidemic was rather late and lasted during 18 weeks. Totally 1433 samples from hospitalized patients were tested and the number of influenza positive cases accounted for 39%, caused by shedding of influenza B/Yamagata-like lineage, A(H3N2) and A(H1N1)pdm09. Only four cases of B/Victoria-like lineage were found. The main target groups for influenza kept pregnant women, elderly people and schoolchildren. Efficacy of vaccine was to 44%. Two deaths, one of them caused by A(H3N2) virus, and 6 severe cases during the season were founded.
Publications and presentations in 2017-2018
- Lvov D.K., Burtseva E.I., Kirillova E.S. et al. Drift of influenza A(H3N2) virus: biological, antigenic and genetic properties in epidemic season 20162017 in Russia and countries of the northern hemisphere.// Problems of virology, 2018; 63 (2), P.61-68.
- Burtseva E.I., Trushakova S.V., Kisteneva L.B.//Pregnancy as a high risk factor to be infected by influenza viruses. 2nd International Meeting of Respiratory Pathogens (IMRP) Conference, 7-9 March 2018, Singapore. Poster presentation.
- Trushakova S.V., Krasnoslobodtsev K.G., Mukasheva E.A. et al. Moscow site activity in GIHSN, 2012-2018.// Scientific Conference "Infectious diseases and antimicrobial means", 4-5 October 2018, Moscow, Oral presentation.
- Burtseva E.I. Influenza vaccines in Russian Federation: experience and efficacy.// 2018 World Life Science Conference, 27-29 October, 2018, Beijing, China. Oral presentation.