CLINICAL REPORT, December 2016 – May 2017 agreement «Global influenza hospital-based surveillance network” - Branch: MOSCOW, Russian Federation
Ministry of HEALTH OF Russian Federation
D.I. Ivanovsky Institute of Virology FSBI “N.F. Gamaleya NRCEM”, Moscow, Russian Federation
CLINICAL REPORT, December 2016 – May 2017
agreement
«Global influenza hospital-BASED surveiLLANCE network” - Branch: MOSCOW, Russian Federation
Address: Gamaleya street 18, 123098, Moscow, Russia
Tel.: +7 (499) 190-3046
Fax: +7 (499) 190-2867
e-mail: elena-burtseva@yandex.ru; elena_burceva@mail.ru
Director
FSBI “N.F. Gamaleya NRCEM” MH RF,
academician RAS Gintsburg A.L.
Responsible person
Chief of influenza etiology and
epidemiology laboratory Burtseva E.I.
The Global Influenza Hospital-based Surveillance Network (GIHSN) is a public-private partnership constituted by an international network of hospitals following the same core protocol to achieve a better understanding of global influenza epidemiology. Last five years (2011-2015) this partnership was supported by the Centro Superior de Investigación en Salud Pública (CSISP), Sanofi Pasteur and Fondation Merieux; during seasons 2015-2017 – by the Foundation for Influenza Epidemiology, France.
FSBI “D.I. Ivanovsky Institute of Virology” Ministry of Health of Russian Federation as a scientific research organization in the field of virology and epidemiology of influenza, National Influenza center (NIC) collaborating with WHO, was involved in GIHSN during 2012-2015. In October 15, 2014 it has been jointed to FSBI “Institute of Microbiology and Epidemiology named after N.F. Gamaleya” Ministry of Health of Russian Federation (Order of Ministry of Health of RF from 17 May 2014 N.220) and since this time it is in structure of FSBI “Federal Research Center of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya” (IIV “N.F. Gamaleya FRCEM”) Ministry of Health of Russian Federation. Since 12 July 2017 FSBI “N.F. Gamaleya FRCEM” was renamed on FSBI “National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya” (“N.F. Gamaleya NRCEM”).
Study setting and population. The study took place in one from four hospitals for infectious diseases in Moscow, FBIH “Clinical Hospital for Infectious Diseases №1” of Health Department of Moscow (CHID), which collaborates with IIV “N.F. Gamaleya NRCEM” in studies of influenza evolution, biological and genetic properties to make recommendation for vaccines and antivirals efficacy. It covers about 15 million citizens and guests of Moscow. CHID specializes on virus infectious diseases mainly, which are caused by influenza and non-influenza respiratory viruses, enteroviruses, hepatitis B and C, rotavirus, AIDS and etc. CHID presents its service (diagnostic, treatment) to 27-30 thousands of patients a year. It has 706 beds, 38% from all beds for patients with infectious diseases in Moscow. The wards for pregnants (69 beds) with virus infections and 12 beds in intensive unit care (IUC) are the most important advantages of this hospital. It is located not far from IIV “N.F. Gamaleya NRCEM”, that allowed to transport all specimens daily, in proper conditions and in time.
Sample management and laboratory procedures. A nasopharyngeal and a pharyngeal swabs (for subjects 14 years old or older) or a nasopharyngeal and a nasal swabs (for subjects less than 14 years of age) will be obtained from each included patient and were sending immediately to IIV “N.F. Gamaleya FRCEM”, where it was tested during 24-48 hours or frozen at -20ºC (for probes which were taken during weekend).
Commercially available RT-PCR assays (Russia) were used to detect influenza A (subtypes H3 and H1pdm09) and influenza B (Yamagata and Victoria lineages) viruses in swabs: AmpliSens Influenza virus A/B-FL (-Eph) (A and B), AmpliSens Influenza virus A-type-FL (H1N1 and H3N2), AmpliSens Influenza virus А/H1-swine-FL (H1N1pdm09). Part sequencing of hemagglutinin (HA) was made for some influenza A-positive samples for which subtyping on A/H1pdm or A/H3 was not be successful. Evolutionary lines for B virus will be detected by part sequencing using primers, made by DNA-technology, Moscow.
The isolation of epidemic strains was made from influenza-positive swabs using MDCK (influenza B virus) and MDSC-SIAT1 (influenza A(H3N2) virus). To study of antigenic properties HI test with reference sera to recommended vaccine strains of 2016-2017 epidemic season composition was used.
Limit number (from vaccinated and unvaccinated) of sera were studied in HI test to detect the level of specific antibodies to both of vaccine and epidemic strains during the first days of hospitalization.
Study process and results.
6 wards for respiratory diseases (316 beds) took part in this study and patients hospitalized in these wards were monitored three days a week – Tuesday, Wednesday and Thursday; there were: 120 from 485 beds for adults, 113 (53 – from 0 to 3 y.o. and 60 – from 3 to 14 y.o.) from 231 beds for children, all 69 beds for pregnants an all 12 beds for patients in intensive care wards. Age of hospitalized patients ranged from 0 to 90 y.o. Patients were interviewed face to face according Protocol of study on the first day of their admitting in the wards.
The study covered 6-months period. First patient was included on week 50, 2016 (December, 2016). Last patient was included on week 18 (May, 2017). During studied period 2244 hospitalized patients were screened.
Exclusion criteria suggested: ILI within more than last 7 days ( ), not ILI ( ), hospitalized in the last 30 days ( ), not able to communicate ( ), no given the consent ( ), non-resident ( ), institutionalized ( ) and others reasons ( ). Totally, 610 (27%) hospitalized patients were excluded from study according of protocol.
Study Protocol suggests including the patients according some criteria. The first one, patients with acute respiratory diseases and diagnoses possibly associated with an influenza infection (ILI). Inclusion criteria also suggested: ILI within the last 7 days with at least one of the following four systemic symptoms: fever, headache, myalgia or malaise and at least one of the following three respiratory symptoms: cough, sore throat or shortness of breath. Totally 1634 (73%) patients were included in study according of protocol (Pic1).
The maximum number of hospitalized patients a week was found during week 4, 2016 (165), meantime the highest number of positive cases (70%) was found during week 3, 2016 (101 from 145).
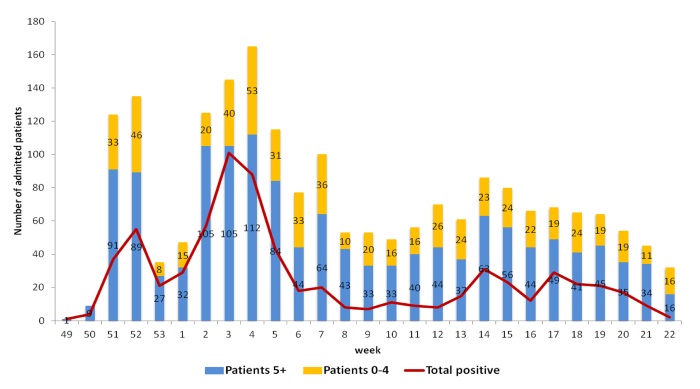
Pic.1. Admission of hospitalized patients during 2016-2017, Moscow
The weekly dynamics of detection of influenza A(H3N2) and B (Yam- and Vic-lineage) infection in clinical materials of hospitalized patients is presented on Pic.2. The epidemic has started due to sharp activity of influenza A(H3N2) virus since the end of December 2016, followed circulation of influenza B in the middle and the end of the season. Influenza A(H3N2) virus was detected in sporadic cases through all season. The peak of epidemic activity was at 3-4 weeks according to the highest hospitalizations at that time. Totally 698 probes (35%) from all studied those were positive of influenza. The frequency of their detection was equal to: A(H1N1)pdm09 – 67,9%, A(H3N2) – 6,6%, B – 22,9%. Almost all of influenza B virus cases (159 from 160) were belonged to influenza B/Vic-lineage.
The different aged groups were included in study, among them: children under 4 y.o – 327, children 5-14 y.o. – 149, adults 15-64 y.o – 1124 (including pregnants – 805) and elderly (65 y.o. and older) – 34 (Pic.3).
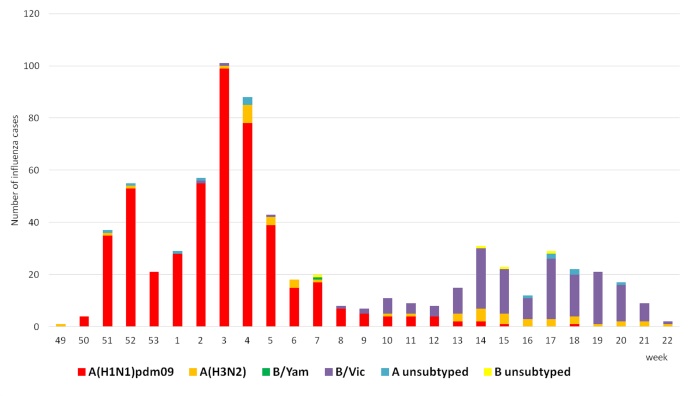
Pic.2. Weekly PCR results for influenza virus detection in clinical materials of patient admitted in study, 2016-2017
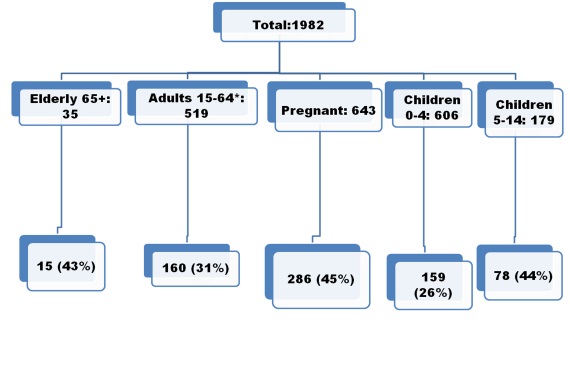
Pic.3. Frequency of influenza positive cases in different aged groups of hospitalized patients including in study during 2016-2017
Totally influenza A(H3N2) virus was detected in all aged groups and percentages of positive cases were from 47,0 (in children of 0-4 y.0) to 81,0 (in elderly) (Pic. 4). Meantime influenza B virus was detected more frequently in age group of children 5-14 y.o. (48,0%).
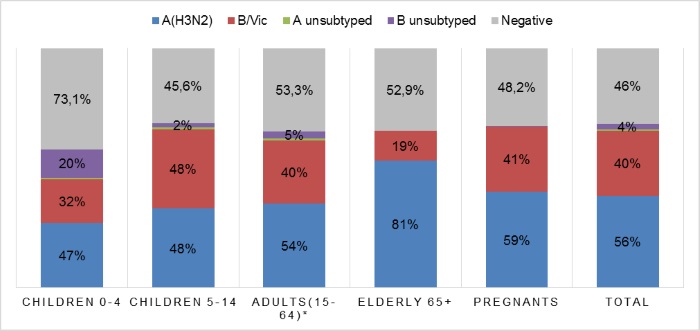
Pic.4. Influenza virus type/subtype distribution in hospitalized patients of different aged groups during 2016-2017
On Pic.5 the results of influenza virus detection in patients in regards to risk factors are presented. Chronic cardiovascular diseases (CVD) were the most common condition in hospitalized adults and elderly patients. More than 40% of these patients had an influenza infection. Renal impairments and autoimmune diseases were the risk factors for pregnants. Moreover autoimmune diseases were met in pregnants mostly.
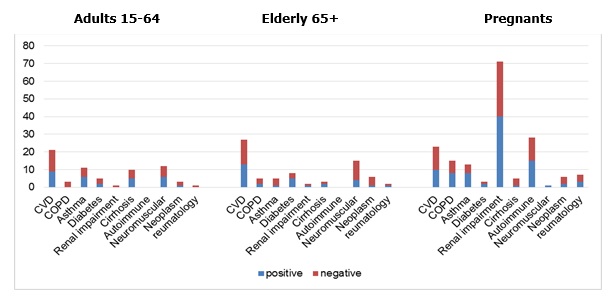
Pic.5. Influenza virus detection in patients with comorbidities during 2016-2017
459 hospitalized patients had bronchitis and in 207 (45%) of them influenza virus infection was detected (Pic.6). The most cases of bronchitis were found in aged groups of patients of 5-64 y.o. with influenza virus infection (60%) and in children of 0-4 y.o. – with other ARVI (75%).
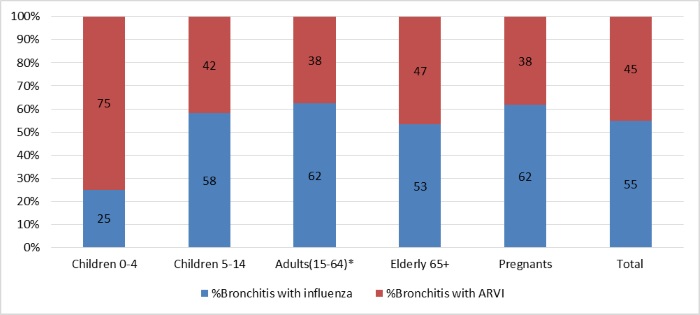
Pic.6. The frequency of bronchitis as complication of influenza and other ARVI in different groups of hospitalized patients during 2016-2017
Pneumonia as a severe complication was diagnosed in 213 hospitalized patients, and in 67 (31%) cases influenza virus infection was found as well: 23% - in group of 0-4 y.o., 11% - in group of 5-14 y.o., 34% - in group of 15-64 y.o. and 46% - in elderly. 48,4% patients had bilateral pneumonia.
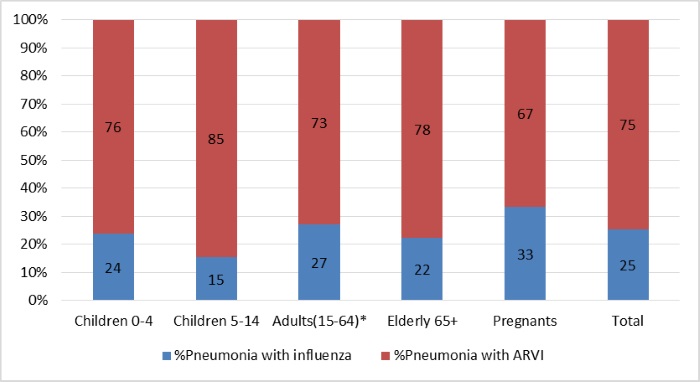
Pic.6. The frequency of pneumonia as complication of influenza and other ARVI in different groups of hospitalized patients during 2016-2017
One of the groups of interest was pregnant women; the frequency of influenza positive cases was 59%. There were not statistical differences in frequency of influenza cases during trimesters: during the first trimester the percentage of influenza cases was 30,8; the second one – 32,4 and the third one – 36,8. Among clinical symptoms there are: fever – 92%, headache – 87%, malaise – 69%, myalgia – 53%, cough – 75%, sore throat – 77% and breath – 19%. The most common complications in pregnants were bronchitis (62%) and pneumonia (31%). Obstetrical pathology among pregnant women with influenza: miscarriage (33%), premature labor (11%) and caesarian labor (15%).
During study period there were patients with severe influenza diseases for which ICU and/or mechanical ventilation were needed: ICU – children 0-4 y.o. – 1, adults 15-64 y.o. - 9, pregnants – 1 and elderly – 1 (total – 12); mechanical ventilation was used for children 0-4 y.o. – 1, adults 15-64 y.o. - 8 and elderly – 1 (total – 10).
In Tabl.1 the results for influenza virus detection in admitted patients in regards to smoking habits are presented. In groups of smokers (21% from all) the positive cases of influenza virus infection were detected less in comparing with never smokers (31,0% accordingly) but there was no any difference in detection of influenza virus typing/subtyping.
Table 1
Results for influenza in patients in regards to smoking habits during 2015-2016
|
Influenza viruses |
Detection of influenza viruses in clinical materials for admitted … |
|
|
Smokers n=560 |
Never smokers n=1113 |
|
|
A(H1N1)pdm09 |
73 |
223 |
|
A(H3N2) |
2 |
30 |
|
A |
6 |
3 |
|
B |
38 |
91 |
|
All positive |
119 ; 21% |
347 ; 31% |
In Tabl.2 the results for influenza virus detection in patients in regards to GP consultations are presented. For aged group of 0-4 y.o. there were differences between influenza positive and negative that may be connect with cases of non-influenza respiratory infections. Meantime in aged group of 5 y.o. and older the number of 3-times GP consultation was differed between groups that may be caused including the big number of pregnants.
Table 2
Results for influenza in patients in regards to hospitalizations and GP consultations
|
Influenza viruses |
Number (%) of patients in regards to GP consultations for aged groups
|
|||||||
|
0-4 y.o. |
5 y.o. and older |
|||||||
|
None
n=56 |
1-2 times n=440 |
3 times n=112 |
All GP (1-3) n=552 |
None
n=600 |
1-2 time n=252 |
3 times n=524 |
All GP (1-3) n=779 |
|
|
All positive
|
15 (27,0)
|
117 (27,0) |
27 (24,0) |
144 (26,0) |
204 (34,0) |
94 (37,0) |
241 (46,0) |
335 (35,0) |
|
Negative
|
41 (73,0) |
323 (73,0) |
85 (76,0) |
408 (74,0)
|
396 (66,0) |
158 (63,0) |
283 (54,0) |
444 (65,0)
|
In Pic.7 the results of detection of influenza virus infection in aged groups of hospitalized patients who were vaccinated by influenza vaccines are presented. In study the total number of vaccinated patients was 69, among them: 0-4 y.o. – 2, 5-14 y.o. – 7, 15-64 y.o – 42, 65 y.o. and older – 5 and pregnants – 11. The most cases of influenza were found in pregnants (55%).
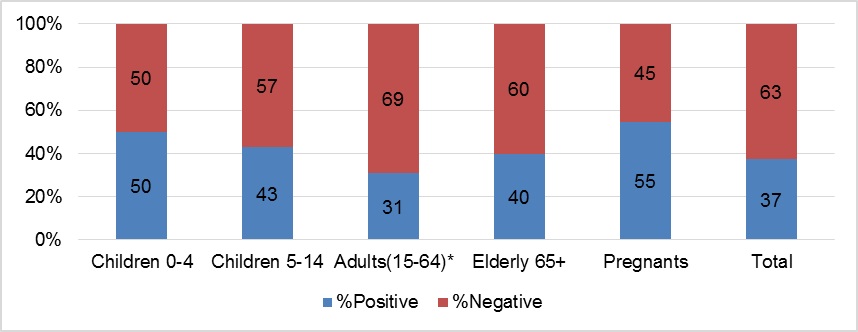
Influenza vaccine effectiveness was estimated as (1 – OR) x 100, where OR compared the vaccine coverage rate between influenza positive and influenza-negative patients. There were difference between the number of influenza positive cases in vaccinated and unvaccinated patients to A(H1N1)pdm09 virus and no that – to A(H3N2) and B viruses; efficacy of vaccine to A(H1N1)pdm09 was to 90%; all patients – 31%.
These differences may be explained by antigenic properties of epidemic strains which were isolated from hospitalized patients.
Table 3
Results of PCR diagnostic of influenza virus infection in vaccinated and unvaccinated
hospitalized patients in 2015-2016
|
Age |
Vaccinated in season 2015-2016 |
Unvaccinated in season 2015-2016 |
||||||||||
|
H1 pdm |
H3 |
B/Vic |
A |
B |
All |
H1 pdm |
H3 |
B/Vic B/Yam |
A |
B |
All |
|
|
0-4 |
2 |
|
1 |
|
|
3/7 |
118 |
4 |
27 |
6 |
1 |
156/599 |
|
5-14 |
2 |
|
1 |
|
|
3/13 |
50 |
2 |
21 |
1 |
1 |
75/166 |
|
15-64 |
3 |
1 |
7 |
1 |
1 |
13/65 |
289 |
36 |
100/1 |
3 |
4 |
433/1097 |
|
65> |
1 |
|
1 |
|
|
2/4 |
9 |
3 |
1 |
|
|
13/31 |
|
All |
8 |
6 |
9 |
1 |
1 |
21/88 |
466 |
45 |
150 |
10 |
6 |
677/1894 |
In IIV NRCEM the HI test with panel of (rat sera and diagnostic sera from WHO kit) was used for antigenic analysis of strains, isolated from clinical materials taking from hospitalized patients: 178 strains of A(H1N1)pdm09, 33 – A(H3N2) and 83 – B virus.
All tested strains of influenza A(H1N1)pdm09 were characterized as A/California/7/2009(H1N1)pdm09-like, which was a component of the 2015-2016 influenza vaccine for the Northern Hemisphere.
All influenza A(H3N2) viruses tested were characterized as A/Hong Kong/5738/2014, which reacted with specific serum till homological titer and differed from the influenza A(H3N2) component of the 2015-2016 influenza vaccine for the Northern Hemisphere (A/Switzerland/9715293/2013); strains reacted with serum to Switzerland/9715293/13 till 1/4 -1/16 of homological titer.
82 from 83 strains of influenza B strains had low reaction with serum to reference strain B/Massachusetts/2/2012 (B/Yamagata lineage), the influenza B component of the 2015-2016 influenza vaccine for the Northern Hemisphere and had good interactions with serum to B/Brisbane/60/2008-like (Victoria lineage). 1 strain reacted with serum to B/Massachusetts/2/2012 (B/Yamagata lineage) till 1/4 of homological titer.
Also we received some difference in results of level of specific antibodies to circulating influenza strains – A(H3N2) and B in sera taking from vaccinated (14) and unvaccinated (79) hospitalized patients (Table 4).
Table 4
Specific antibodies (HI test) to influenza viruses in sera taking during hospitalization of patients with acute respiratory disease in 2016-2017
|
Influenza viruses |
Vaccinated (6) |
Unvaccinated ARVI (18) / SARI (11) |
||
|
GMT±2m (lg) |
≥1:40 (%) |
GMT±2m (lg) |
≥1:40 (%) |
|
|
A/California/07/2009 (H1N1)pdm09 – v* |
5,7±2,0 |
67,0 |
4,3±0,9 / 3,7±0,7 |
39,0 / 27,0 |
|
A/Michigan/45/2015 (H1N1)pdm09 |
5,0±1,5 |
50,0 |
4,2±0,9 / 3,9±1,1 |
33,0 / 27,0 |
|
A/Switzerland/9715293/2015 (H3N2) |
6,2±1,3 |
83,0 |
4,7±0,9 / 4,0±1,0 |
50,0 / 36,0 |
|
A/Hong Kong/5738/14 (H3N2) – v* |
7,3±0,9 |
100 |
4,8±1,1 / 3,7±0,9 |
44,0 / 18,0 |
|
B/Phuket/3073/13 (Yam) |
7,0±1,3 |
100 |
5,9±0,9 / 5,6±1,3 |
83,0 / 82,0 |
|
B/Brisbane/60/2008 (Vic) – v* |
6,0±1,8 |
67,0 |
4,9±0,8 / 4,6±0,9 |
39,0 / 27,0 |
A/California/07/2009 (H1N1)pdm09 – v* - vaccine strain
We received some difference in results of level of specific antibodies to circulating influenza strains – A(H1N1)pdm09, A(H3N2) and B in sera taking from vaccinated (6) and unvaccinated (with ARVI - 18, with SARI - 11) hospitalized patients (Table). The results of GMT indicated differences between the data of specific antobodies to both A(H3N2) viruses (vaccine and epidemic) virus. Also there were differences in number of patients with seroprotection level of antibodies to all circulating viruses between vaccinated and unvaccinated patients. These data may confirmed the efficacy of vaccination. Among 6 vaccinated patients the influenza virus infection was not be found, meantime in unvaccinated – 2 cases of influenza A(H3N2) virus infection was found in patients with SARI.
Key aspects from the season
Sharp start of influenza season on 49-50 wks of December 2016
Dominant influenza virus – A(H3N2)
No one case of influenza A(H1N1)pdm09 and B/Yamagata-lineage
Bronchitis are commonly registered during influenza infection
CVD, renal and autoimmune diseases are most common and contribute to influenza infection
Prevalence of pregnant women among female admissions
A great number of pregnant women with influenza (more 50%)
No deaths and 1 case of ICU
Challenges
A lot of exclusions (27%)
Few vaccinated patients
Some challenges in gathering data from vaccinated patients
Challenge in patients needed ICU
In comparing with previous seasons: number of patients was increased, percent of positive on influenza was higher, sufficient group of pregnant women and children
Weakness: low number of vaccinated, group of elderly and no activities in diagnostic of other respiratory viruses only for sporadic
All data received during study period according Questionnaire were presented in Excel table in June 2016.